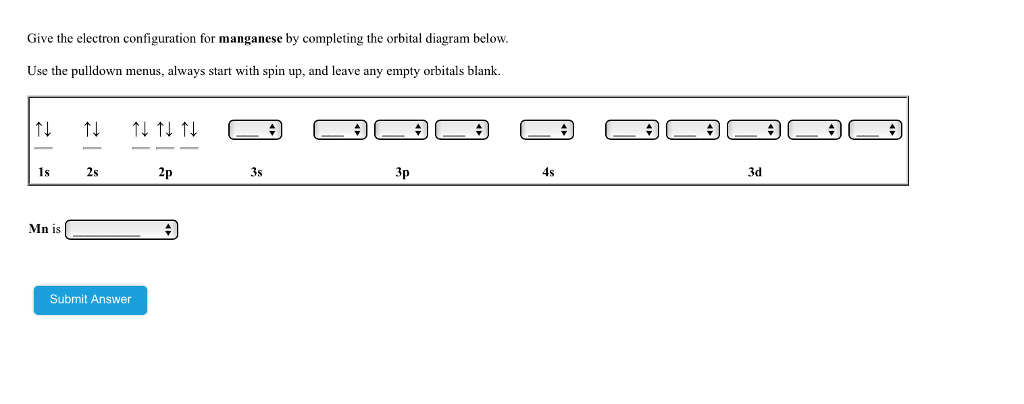
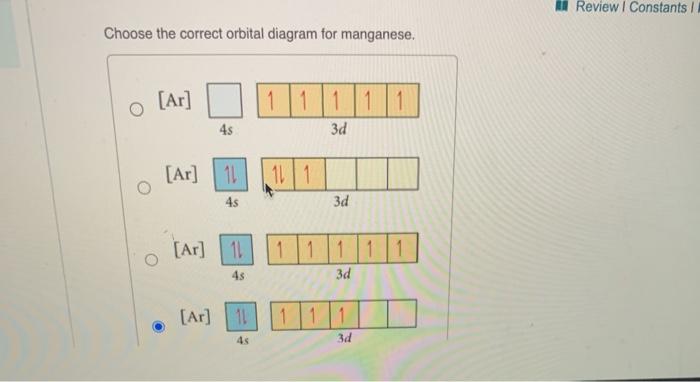
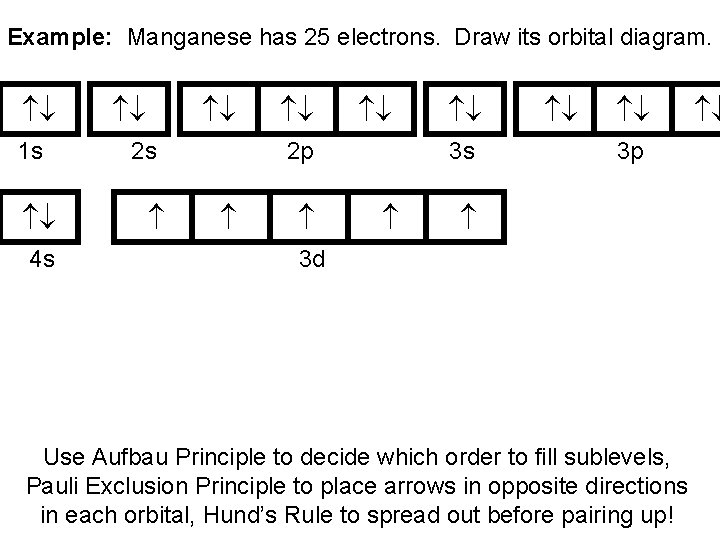
39 orbital diagram for manganese
Hey! I’ve recently started studying chemistry as one of my university classes. I’m genuinely curious, is the definition of paramagnetism and diamagnetism a bit problematic? From definition, paramagnetic substances are those with unpaired electrons. The opposite goes with diamagnetic substances, whose electrons are all paired. I see that some elements follow this, like Manganese for example. It contains unpaired electrons and is thus paramagnetic. Why is it though that other elements don’t fo... Nov 01, 2021 · Orbital diagram of Manganese (Mn) 26: Orbital diagram of Iron (Fe) 27: Orbital diagram of Cobalt (Co) 28: Orbital diagram of Nickel (Ni) 29: Orbital diagram of Copper (Cu) 30: Orbital diagram of Zinc (Zn) 31: Orbital diagram of Gallium (Ga) 32: Orbital diagram of Germanium (Ge) 33: Orbital diagram of Arsenic (As) 34: Orbital diagram of Selenium ...
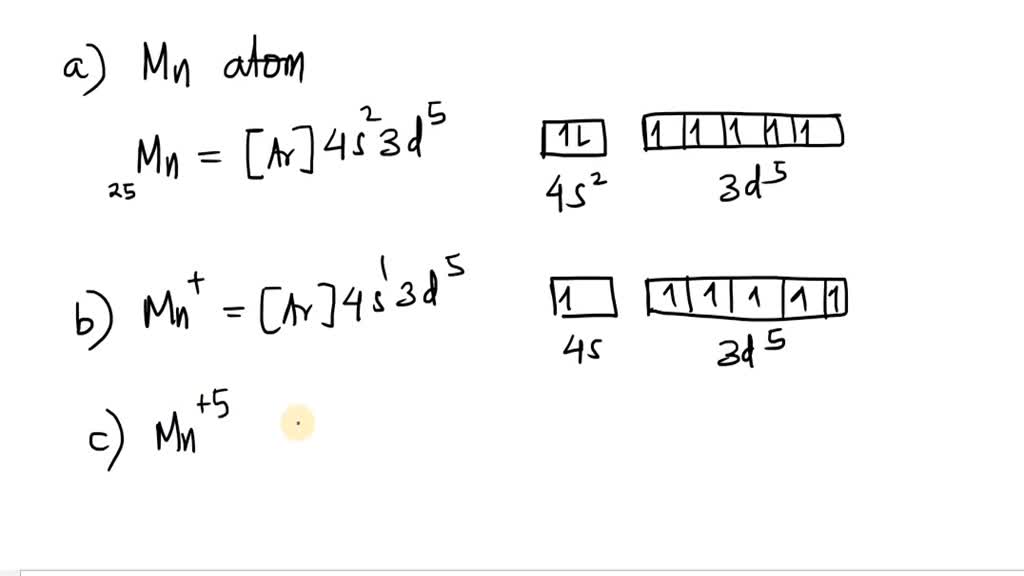
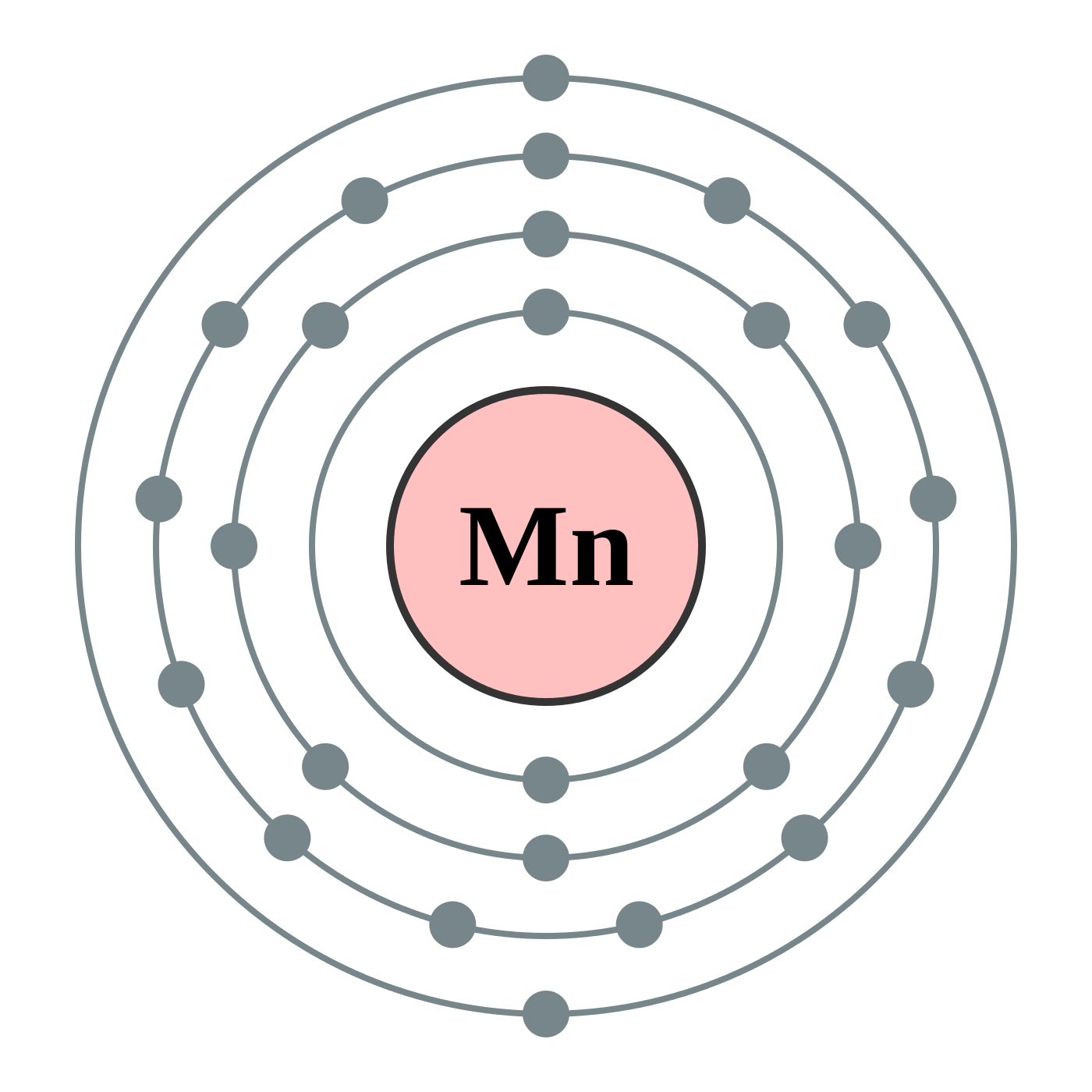
29 Jul 2016 — The electron configuration of manganese, atomic number 25, is 1s2222p63s23p63d54s2 . The diagram below represents the electron configuration ...1 answer · Refer to the explanation. Explanation: The electron configuration of manganese, atomic number 25, is 1s2222p63s23p63d54s2. The diagram below represents ...

Orbital diagram for manganese
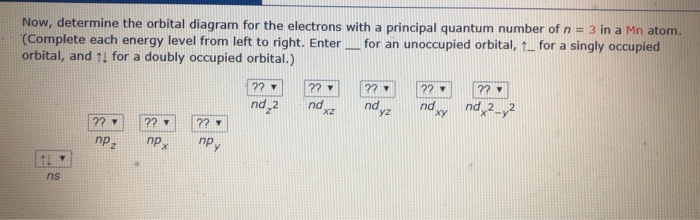
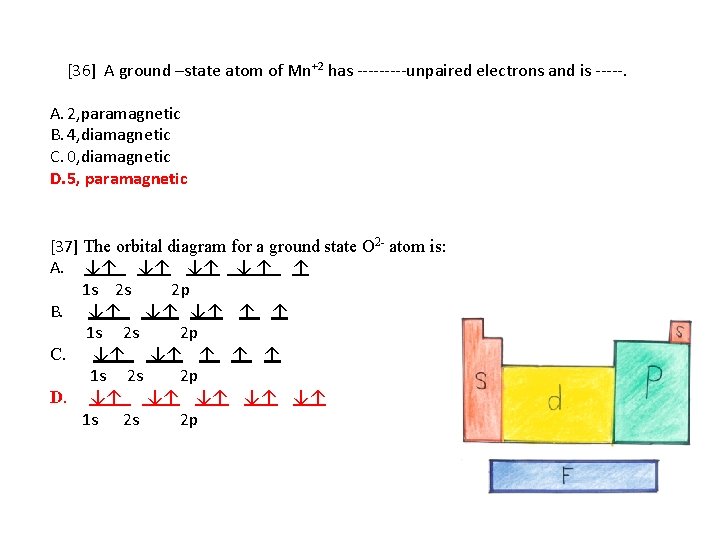
So, in class we did a lab comparing the magnetic properties of the first row of transition elements (From Manganese to Zinc) as cations (all 2+), and one of the post-lab questions talks about the electron configuration and orbital diagram of each cation. I understand that for each one, the 4s orbital electrons will go away, rather than the 3d orbital electrons, but Copper is an exception. It's electron configuration is [Ar] 4s1 3d10, rather than [Ar] 4s2 3d9. But what about Cu2+? It's electron c... Version 2.0 and Part 7 of "Utopian Religion", "Utopia", or "Vispthinkingpat, Thinkflexsense, and Soundpat Religion" &#x200B; # Efficient vispthinkingpat combination of English language, numerical list format, and logic language or Vispenlogist Language: * Listology <-> List-types-ology <-> Indented-list-ology and Non-indented-list-ology and Numerically-ordered-list-ology and Bulletpoint-ordered-list-ology and Vispenlogistology * Combatology <-> Shield-from-enemy-ology... So if you look at the periodic table, Manganese is going to have an electron configuration of one S 2. She was too two P six, three S two, three, P six 45. And ...1 answer · Top answer: You can determine the ground-state electron configuration of Manganese (Mn) by referring to the periodic table and locating the position of Mn in the ...
Orbital diagram for manganese. Manganese (Mn) has an atomic mass of 25. Find out about its chemical and physical properties, states, energy, electrons, oxidation and more. ... Orbital Diagram. 1s ... Feb 08, 2015 · Explanation: Oxidation States. +2,3,4,6,7. Electrons Per Shell 2 8 15. Electron Configuration [Ar] 4s2 3d5. 1s2 2s2 2p6 3s2 3p6 4s2 3d5. Answer from: angelinaissoocp33868. SHOW ANSWER. The correct answer is Option D. Explanation: Orbital energy diagrams showing the possible ways that Mn(IV) can add two electrons to its e * g orbital set. Accepting two electrons in one orbital is more ... 11 Apr 2021 — The ground state electron configuration of ground state gaseous neutral manganese is [Ar]. 3d5. 4s2 and the term symbol is 6S5/2.
Draw the orbital diagram diagram for manganese. manganese mn chemicalaid manganese mn has an atomic mass of 25 find out about its chemical and physical properties states energy electrons oxidation and more. Hf Molecular Orbital Diagram – Orbital Diagram For Fluorine Awesome 0d Mos2 2d G. arrangements of electrons in the orbitals of an atom is the orbital diagram the electron configuration and the energy diagram all three ways are useful the next atom is helium with 2 electrons so the second electron could go into the 1s orbital with the ... Okay, so for just a neutral magnesium manganese, it would be if we ... Okay, so let's go ahead and draw a orbital diagram for manganese plus ...14 May 2018 So I need someone to check some of these, so I might crosspost this to other subreddits, if you know any, please do. Or if you are an expert yourself, please correct me if there's any mistakes. But I did watch Dr. Stone in an *Anime Streaming website*, I posted some interesting comments in the discussions of Dr. Stone Episodes. I will post them in a chronological order with the matching episodes. Although, I think it's a bad idea to post this in a whole one post. Because no one gonna read it t...
28 Jul 2021 — Electron Configuration for Mn · With the Aufbau principle, the first orbital has 2s, 2p, 3s, 3p, 4s, 3d, 4p, and so on electrons. · Subsequently, ... Mn (Manganese) is an element with position number 25 in the periodic table. Located in the IV period. Melting point: 1244 ℃. Density: 7.44 g/cm 3 . Electronic configuration of the Manganese atom in ascending order of orbital energies: 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 5. So if you look at the periodic table, Manganese is going to have an electron configuration of one S 2. She was too two P six, three S two, three, P six 45. And ...1 answer · Top answer: You can determine the ground-state electron configuration of Manganese (Mn) by referring to the periodic table and locating the position of Mn in the ... Version 2.0 and Part 7 of "Utopian Religion", "Utopia", or "Vispthinkingpat, Thinkflexsense, and Soundpat Religion" &#x200B; # Efficient vispthinkingpat combination of English language, numerical list format, and logic language or Vispenlogist Language: * Listology <-> List-types-ology <-> Indented-list-ology and Non-indented-list-ology and Numerically-ordered-list-ology and Bulletpoint-ordered-list-ology and Vispenlogistology * Combatology <-> Shield-from-enemy-ology...
So, in class we did a lab comparing the magnetic properties of the first row of transition elements (From Manganese to Zinc) as cations (all 2+), and one of the post-lab questions talks about the electron configuration and orbital diagram of each cation. I understand that for each one, the 4s orbital electrons will go away, rather than the 3d orbital electrons, but Copper is an exception. It's electron configuration is [Ar] 4s1 3d10, rather than [Ar] 4s2 3d9. But what about Cu2+? It's electron c...
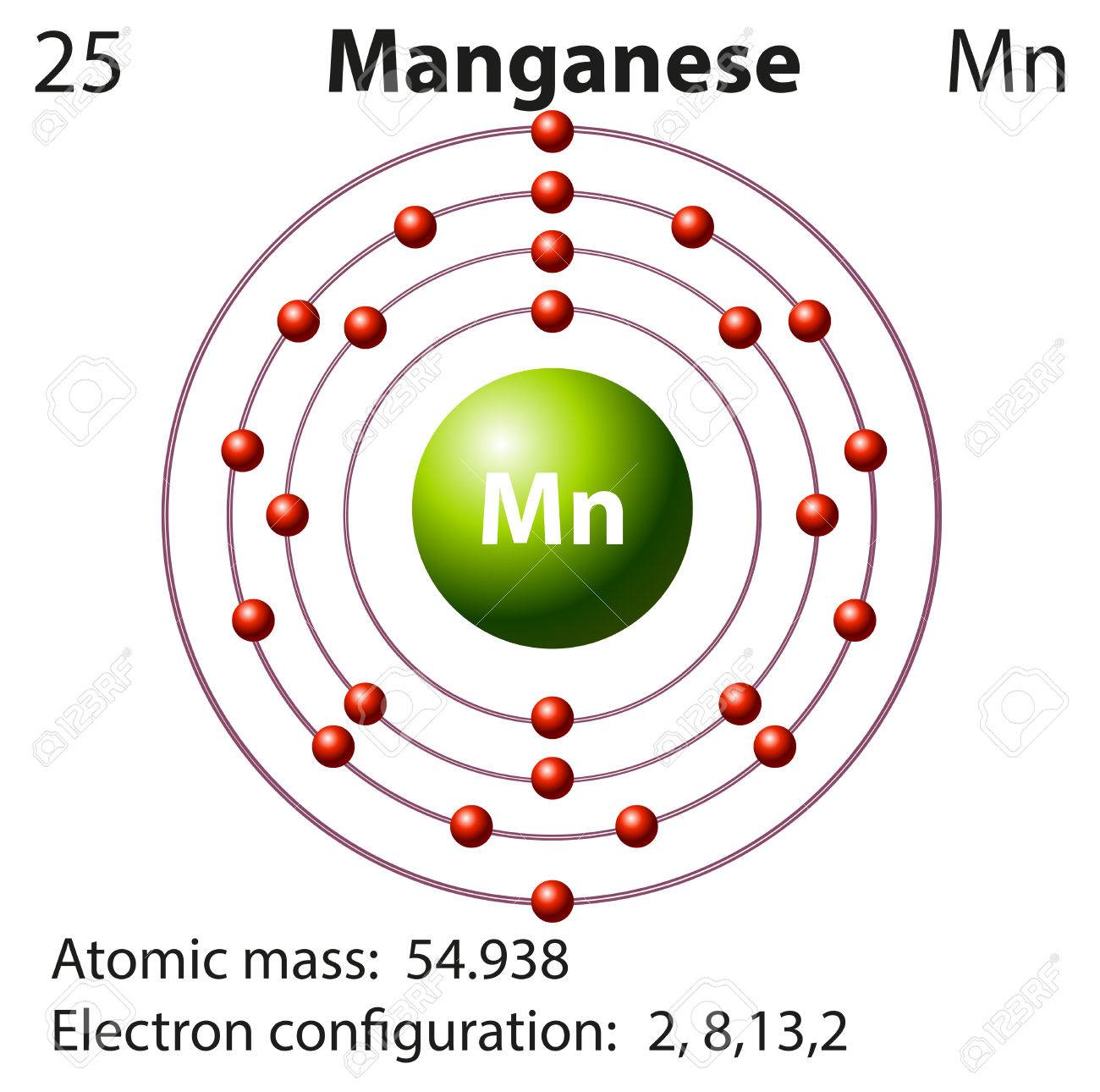
Symbol And Electron Diagram For Manganese Illustration Royalty Free Cliparts Vectors And Stock Illustration Image 46911273

Mn Manganese Element Information Facts Properties Trends Uses And Comparison Periodic Table Of The Elements Schoolmykids
Consider The Molecule Mnx6 2 Where X Is A Neutral Ligand Assume That X Is A Strong Field Ligand Socratic

Electron Configurations How To Write Out The S P D F Electronic Arrangements Of Atoms Ions Periodic Table Oxidation States Using Orbital Notation Gce A Level Revision Notes


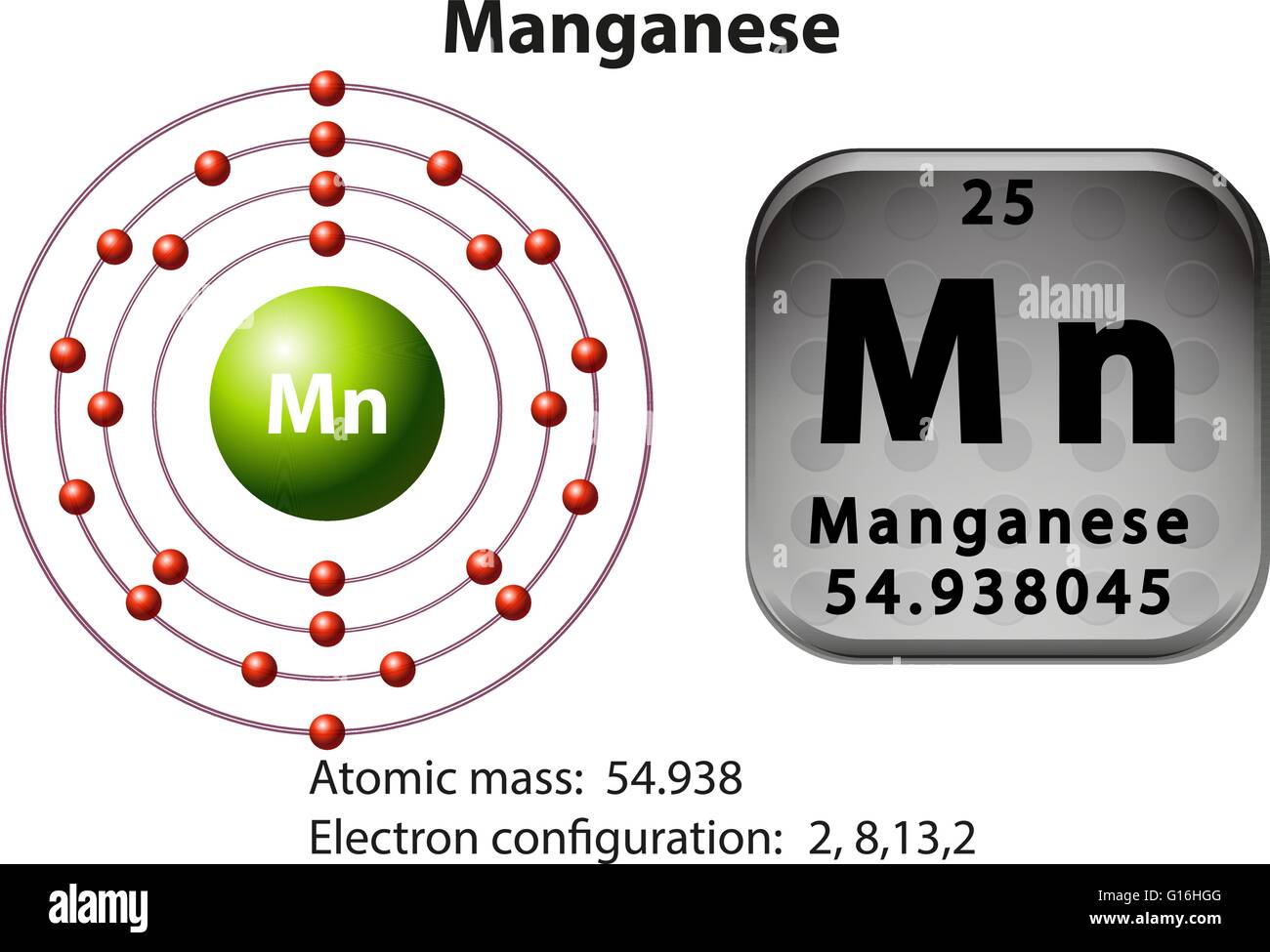
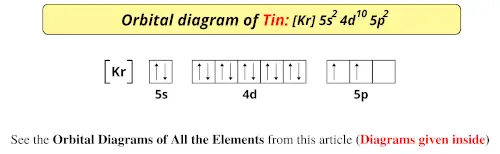
/800px-Orbital_representation_diagram.svg-589bd6285f9b58819cfd8460.png)


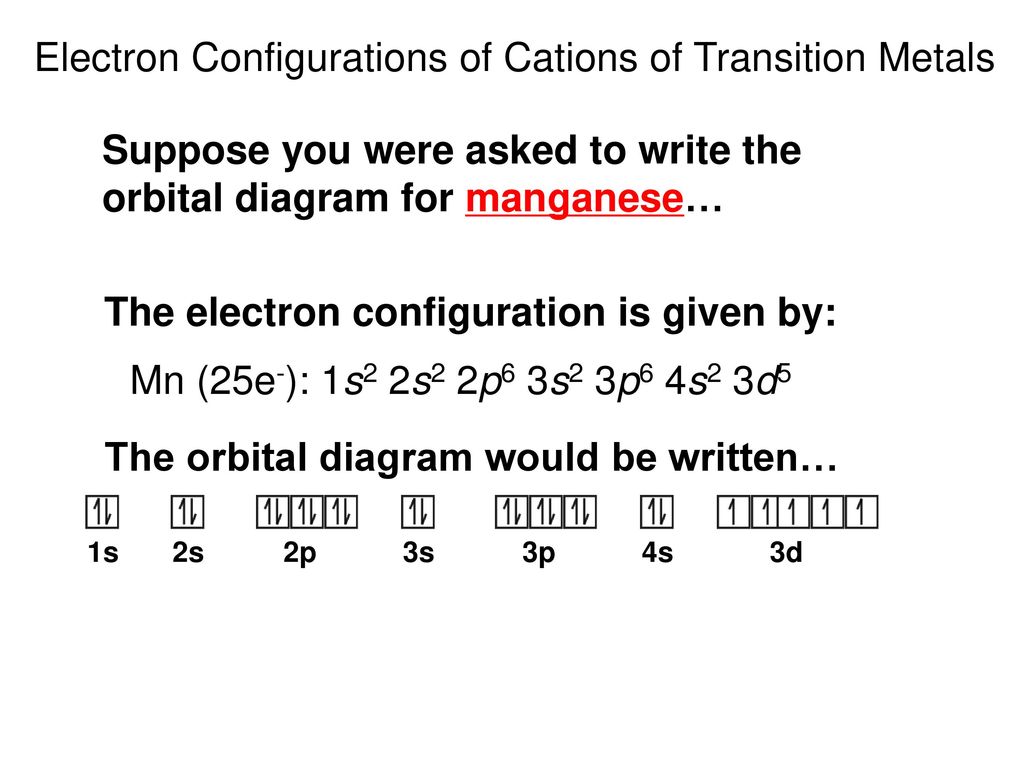



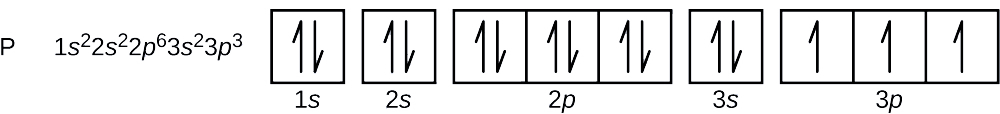






Comments
Post a Comment