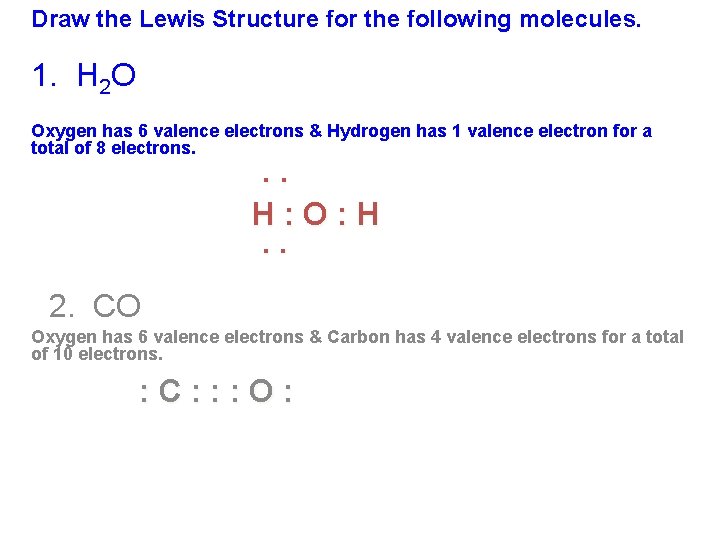
43 carbon monoxide dot diagram
A step-by-step explanation of how to write the Lewis Dot Structure for Carbon Monoxide.For the CO Lewis structure, calculate the total number of valence elec... Lewis Dot of Carbon Monoxide. CO. Back. 70 More Lewis Dot Structures. Produced from the incomplete combustion of hydrocarbons. In the winter, it is important that furnaces have access to an ample supply of oxygen in the air. It is a colorless, odorless, tasteless gas that highly toxic to humans. CO contains a covalent double bond and a third ...
1.12.2021 · Nitrous oxide (N2O) and carbon monoxide (CO) are important drivers in global warming. However, there are many difficulties in reliable monitoring, …

Carbon monoxide dot diagram
A step-by-step explanation of how to draw the Lewis Dot Diagram for CO. Use the periodic table of elements to find the total number of valence electrons for the CO molecule. Once we know how many valence electrons does carbon monoxide have we can distribute them around the central atom with the goal of filling the outer shells of each atom. According to Merriam-Webster and the Online Etymology Dictionary, the word "molecule" derives from the Latin "moles" or small unit of mass.. Molecule (1794) – "extremely minute particle", from French molécule (1678), from New Latin molecula, diminutive of Latin moles "mass, barrier". A vague meaning at first; the vogue for the word (used until the late 18th century only in Latin form) can ... 2.12.2021 · CCl4 is also named carbon chloride, methane tetrachloride, benziform, and more. The liquid is not soluble in water and is non-combustible. The boiling point of CCl4 is 76.8 degrees Celcius and its melting point is -23.0 degrees Celcius. CCl4 will release toxic fumes like …
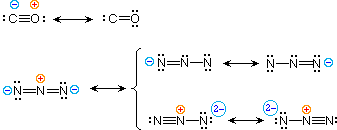
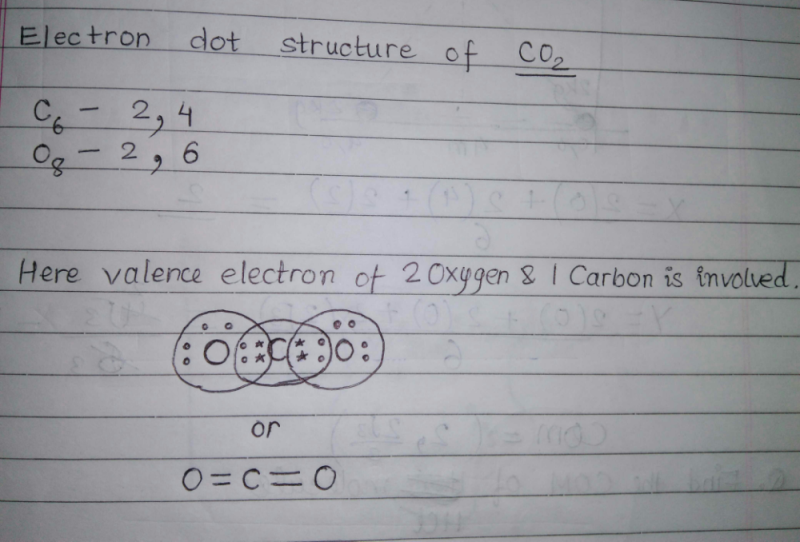
Carbon monoxide dot diagram. Begin finding the chemical formula for carbon monoxide. That is #CO#. Then determine how many valence electrons each element has: Carbon has #4# valence electrons and Oxygen has #6#.In total, there are #10# valence electrons.. I realize that explaining how to draw the lewis dot structure in words might get confusing so what I'm going to do is put a picture of what the diagram will look like ... A step-by-step explanation of how to draw the CO(Carbon Monoxide) Lewis Dot Diagram.For the CO structure use the periodic table to find the total number of v... 15.11.2021 · Lewis Electron Dot Structure for the molecule: CO 2. An oxygen atom has 6 valence electrons and a carbon atom has 4. So carbon shares 2 with one oxygen atom and 2 with the other oxygen atom. Hence two double bonds are formed. Lewis Electron Dot Structure of the molecule: CO (carbon monoxide) The carbon atom has a valency of 4. Answer (1 of 2): Actually a double bond does not satisfy the octet. in the triple bond picture, there is a negative charge on the carbon and a positive on the oxygen. also in the triple bond picture is an oxygen that has three bonds and a lone pair (meaning it owns 5 electrons, but has 8 in its o...
Chapter 5 – Covalent Bonds and Introduction to Organic Molecules Chemical bonds are generally divided into two fundamentally different types: ionic and covalent. In reality, however, the bonds in most substances are neither purely ionic nor purely covalent, but lie on a spectrum between these extremes. In this video, I draw the Lewis dot structure for carbon monoxide. This follows my previous video on the Lewis dot structure for carbon dioxide.https://yout... A video explanation of how to draw the Lewis Dot Structure for Carbon Monoxide, along with information about the compound including Formal Charges, Polarity,... 1. Is Carbon monoxide acidic or basic? Carbon monoxide is a neutral gas since it does not show basic and acidic properties when it reacts with water. 2. Explain CO dot structure in simple words. The overall carbon to oxygen atom ratio in a CO dot structure is 1:1. By sharing three valence electrons, carbon forms three tipple bonds with oxygen.
Nitrogen Monoxide is a paramagnetic gas, also known as nitric oxide.It is colorless and neutral.Its molecule orbital diagram resembles that of carbon monoxide. Electron dot diagram of C2H6? 23.11.2021 · Students observe teacher-led demonstrations, and build and evaluate simple models to understand the greenhouse effect, the role of increased greenhouse gas concentration in global warming, and the implications of global warming for engineers, themselves and the Earth. In an associated literacy activity, students learn how a bill becomes law and they research global warming legislation. Electron dot structure of non-polar covalent molecules on the basis of duplet and octet of electrons (example: hydrogen, chlorine, oxygen, nitrogen, carbon tetrachloride and methane. • Polar Covalent compounds based on – difference in electronegativity: Examples – HCl, NH. 3. and H. 2. O including electron dot structures. • I quickly take you through how to draw the Lewis Structure of Carbon Monoxide. I also go over hybridization and bond angle.
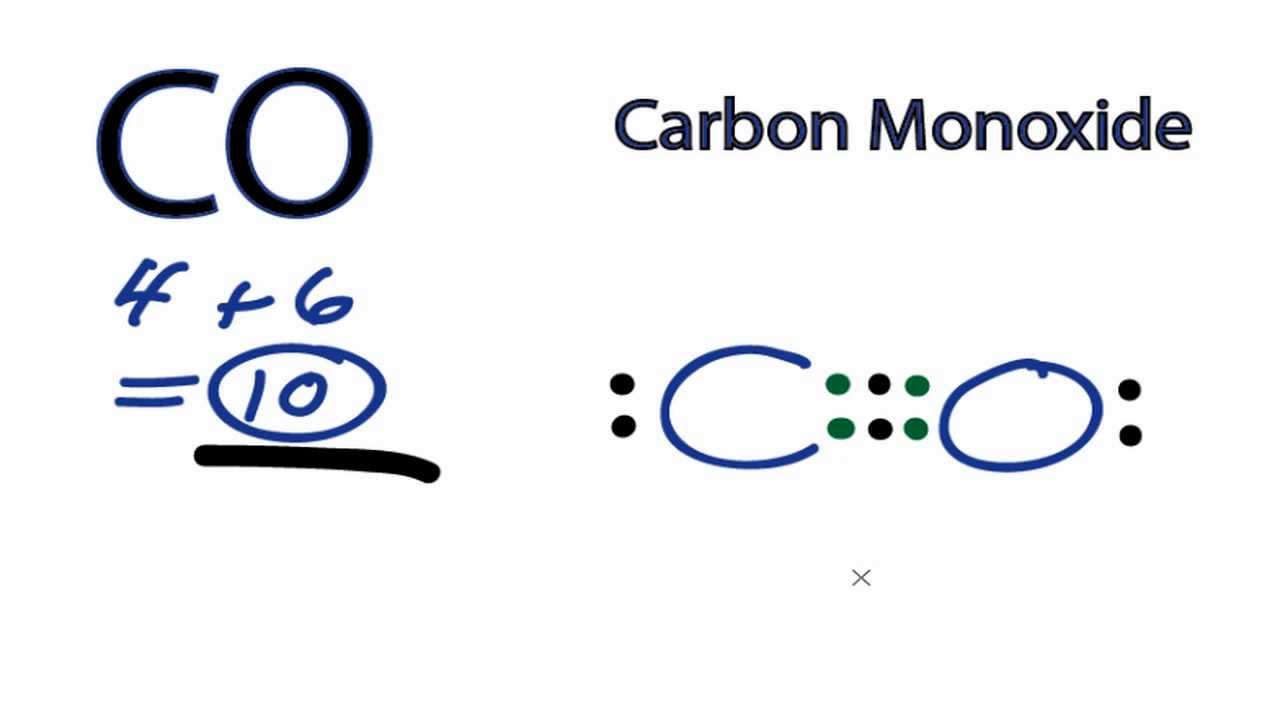
The Lewis structure for CO has 10 valence electrons. For the CO Lewis structure you'll need a triple bond between the Carbon and Oxygen atoms in order to satisfy the octets of each atom while still using the 10 valence electrons available for the CO molecule. YouTube. Wayne Breslyn. 407K subscribers.
The carbon monoxide is produced from the partial oxidation of carbon dioxide (CO2) or any other carbon-containing element. The Lewis structure, also called as electron dot structure, is a simplified method of representing the number of valence electrons present within an atom or a molecule.
8.3.2016 · These impurities consist of carbon as gaseous carbon monoxide, and silicon, manganese, phosphorus and some iron as liquid oxides, which combine with lime and dolime to form the steel slag. At the end of the refining operation, the liquid steel is tapped (poured) into a ladle while the steel slag is retained in the vessel and subsequently tapped into a separate slag pot.

Draw The Lewis Structure Of Co Include Lone Pairs And Formal Charges Please Dont Forget The Homeworklib
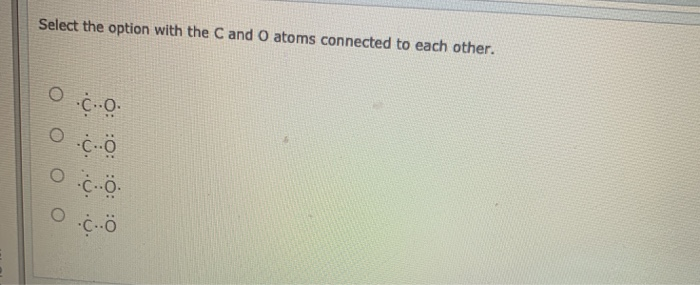
Table 4-1. Chemical Identity of Carbon Monoxide Characteristic Carbon monoxide Synonym(s) Carbon oxide, flue gas, monoxide Registered trade name(s) Chemical formula COb Chemical structure :C O: Identification numbers: CAS registry 630-08-0b NIOSH RTECS FG350000 EPA hazardous waste No data OHM/TADS No data DOT/UN/NA/IMDG shipping 1016c; 9202d,e

Lewis Structure Of Co Carbon Monoxide Carbon Monoxide Lewis Dot Structure Co Lewis Structure Youtube
Learn how to use the MQ-7 carbon monoxide sensor with Arduino and other microcontrollers. The MQ-7 is one of the MQ group of gas sensors which are easy to …
Transcript: This is the CO Lewis structure: Carbon monoxide. We have 4 valence electrons for Carbon and 6 for Oxygen, for a total of 10 valence electrons. So we have a Carbon and an Oxygen atom bonded together. We'll put 2 electrons between the atoms to form a chemical bond, that's 2; and then around the outer atoms, that's 4, 6, 8, and 10.
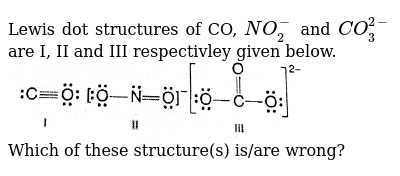
Lewis Dot Structures Of Co No 2 And Co 3 2 Are I Ii And Iii Respectivley Given Below Img Src Https D10lpgp6xz60nq Cloudfront Net Physics Images Bit Chm C04 E01 012 Q01 Png Width 80 Which Of These Structure S Is Are Wrong
Carbon monoxide is a one-carbon compound in which the carbon is joined only to a single oxygen.It is a colourless, odourless, tasteless, toxic gas. It has a role as a neurotoxin, a signalling molecule, a vasodilator agent, a neurotransmitter, a metabolite, a P450 inhibitor, a ligand, a biomarker, a probe, a human metabolite, a mouse metabolite, an EC 1.9.3.1 (cytochrome c oxidase) inhibitor ...
Carbon tetrachloride is a manufactured chemical that does not occur naturally. It is a clear liquid with a sweet smell that can be detected at low levels. It is also called carbon chloride, methane tetrachloride, perchloromethane, tetrachloroethane, or benziform.Carbon tetrachloride is …
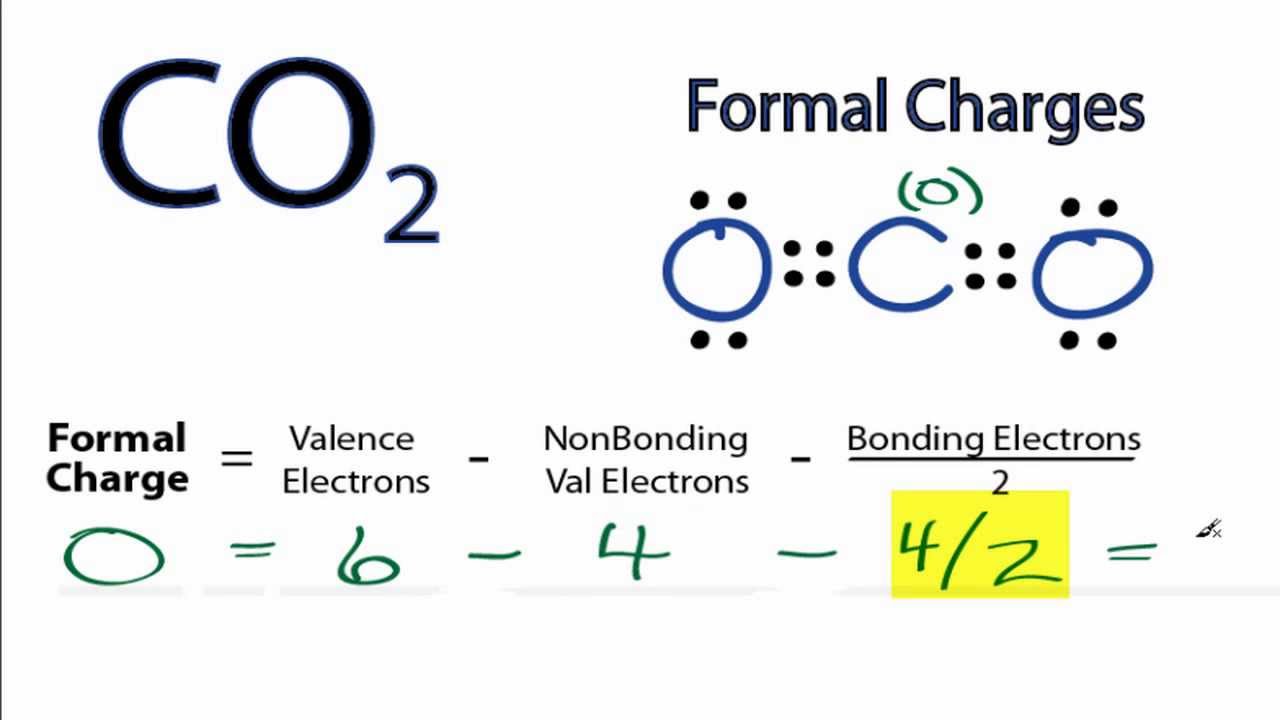
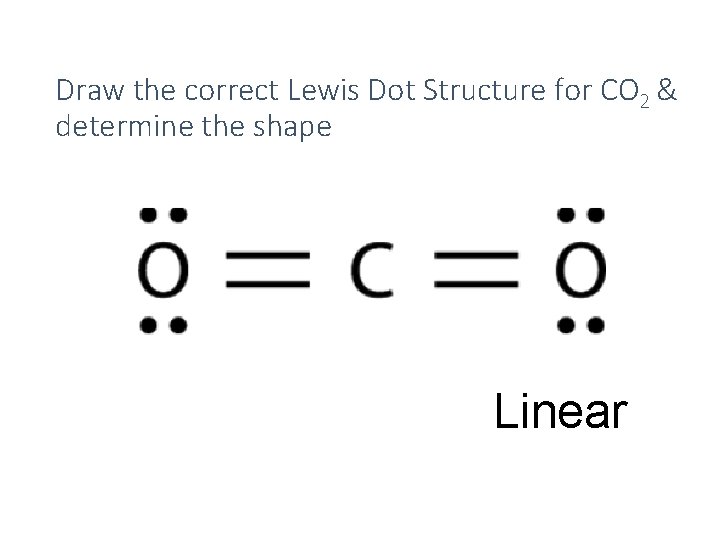
Lewis Structure Examples. The Lewis electron dot structures of a few molecules are illustrated in this subsection. 1. Lewis Structure of CO2. The central atom of this molecule is carbon. Oxygen contains 6 valence electrons which form 2 lone pairs. Since it is bonded to only one carbon atom, it must form a double bond.
Answer: Well,there are some recommendations to follow for determine a Lewis structure,first you must count the number of valence electrons of each atom of the molecule and add them, in this case, we have (carbon= 4e) + (oxygen= 6e)= 10 electrons (this is the number of "dots" that you're going to ...
The Lewis Structure (Lewis Dot Diagram) for CO.1. Count electrons2. Put least electronegative atom in centre3. Put one electron pair in each bond4. Fill oute...
The Lewis Dot Structure (Lewis Dot Diagram) of or for CO.1.Count valence electrons of CO2.Keep the least electronegative atom in centre3.Put one valence e...
2.12.2021 · CCl4 is also named carbon chloride, methane tetrachloride, benziform, and more. The liquid is not soluble in water and is non-combustible. The boiling point of CCl4 is 76.8 degrees Celcius and its melting point is -23.0 degrees Celcius. CCl4 will release toxic fumes like …
According to Merriam-Webster and the Online Etymology Dictionary, the word "molecule" derives from the Latin "moles" or small unit of mass.. Molecule (1794) – "extremely minute particle", from French molécule (1678), from New Latin molecula, diminutive of Latin moles "mass, barrier". A vague meaning at first; the vogue for the word (used until the late 18th century only in Latin form) can ...
A step-by-step explanation of how to draw the Lewis Dot Diagram for CO. Use the periodic table of elements to find the total number of valence electrons for the CO molecule. Once we know how many valence electrons does carbon monoxide have we can distribute them around the central atom with the goal of filling the outer shells of each atom.

How To Draw The Lewis Dot Structure Of Carbon Monoxide Does It Have An Odd Electron Pair Is It An Brainly In






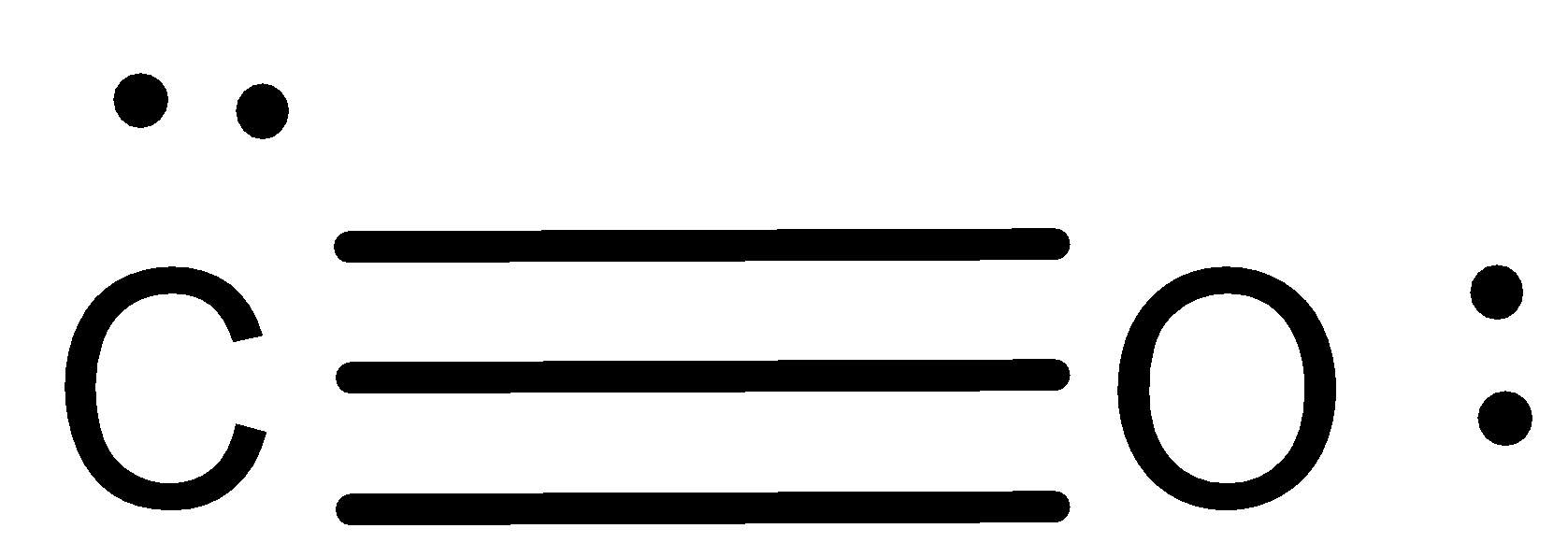





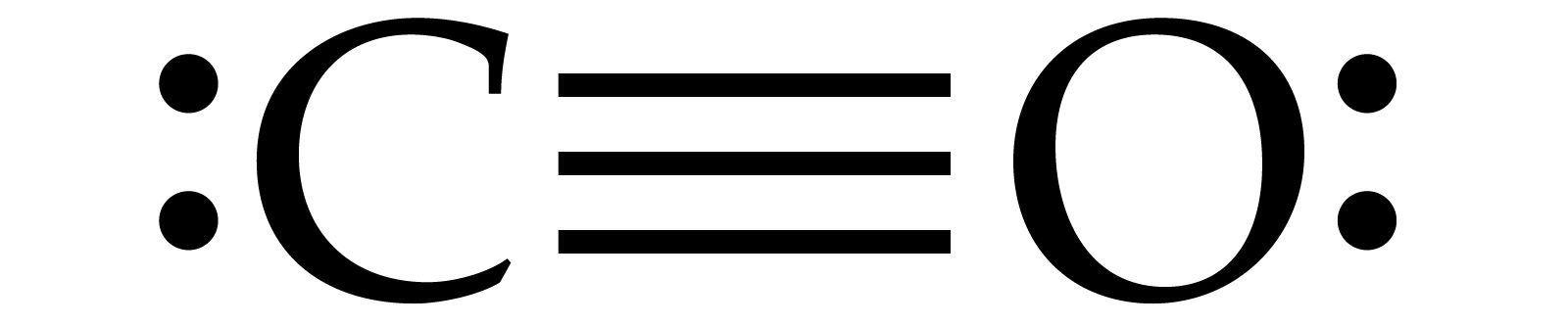







Comments
Post a Comment