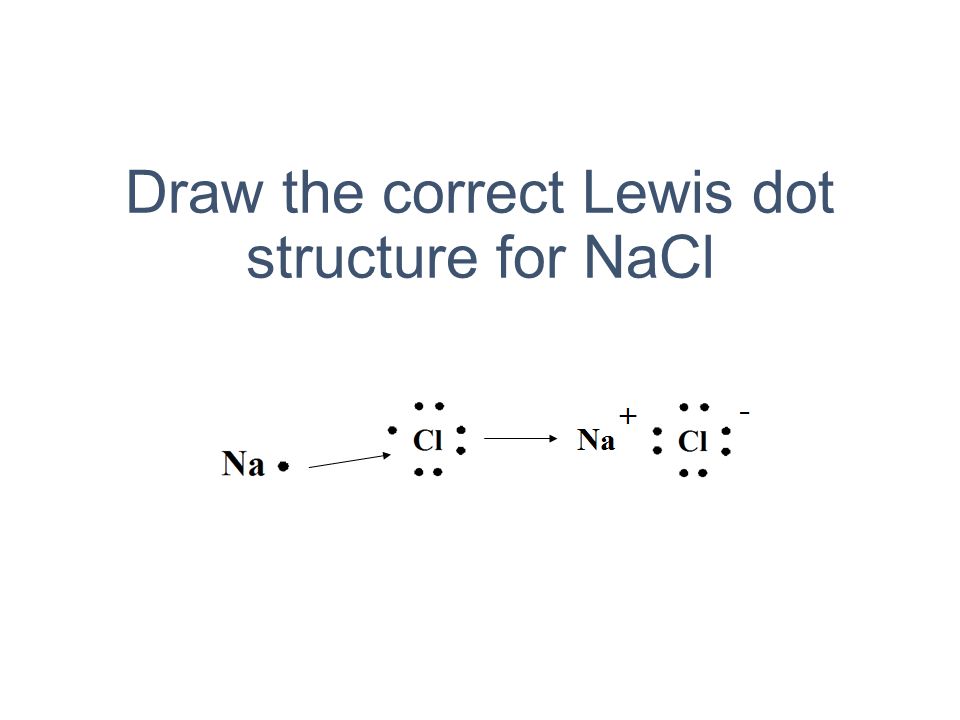
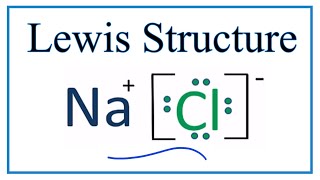
42 lewis dot diagram for sodium chloride
The molecular formula for calcium chloride is CaCl2. You know that calcium is a group 2 element and has two valence electrons. You also know that Cl is a group 7 and has 7 valence electrons. You would take the two available electrons on the Ca, and combine with two Cl atoms. Place two dots around calcium and seven dots around two Cl atoms and ... Draw electron dot representation for the formation of sodium chloride. Solve Study Textbooks. >>. Class 11. >> Chemistry. >> Chemical Bonding and Molecular Structure.
Lewis Dot Diagram. A method for representing an atom's valence electrons using dots around the element symbol. Oxygen (dot diagram) Ca (dot diagram) ... sodium chloride dot diagram. Sodium loses it's one electron. Related questions. QUESTION. There is a relationship between the electron configurations of elements and their chemical and physical ...
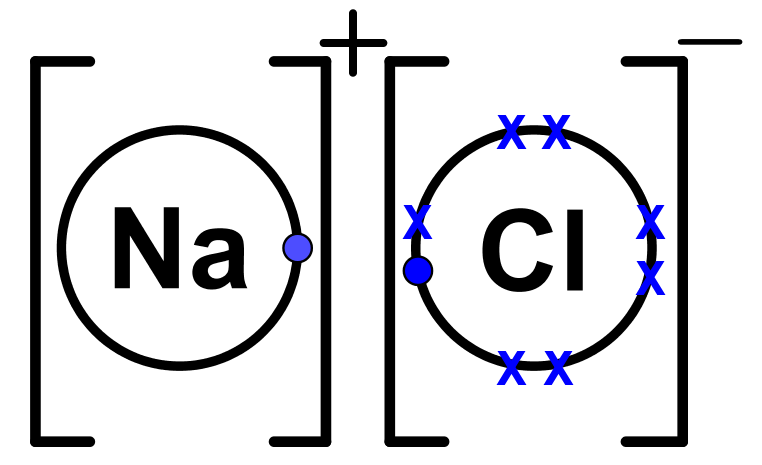
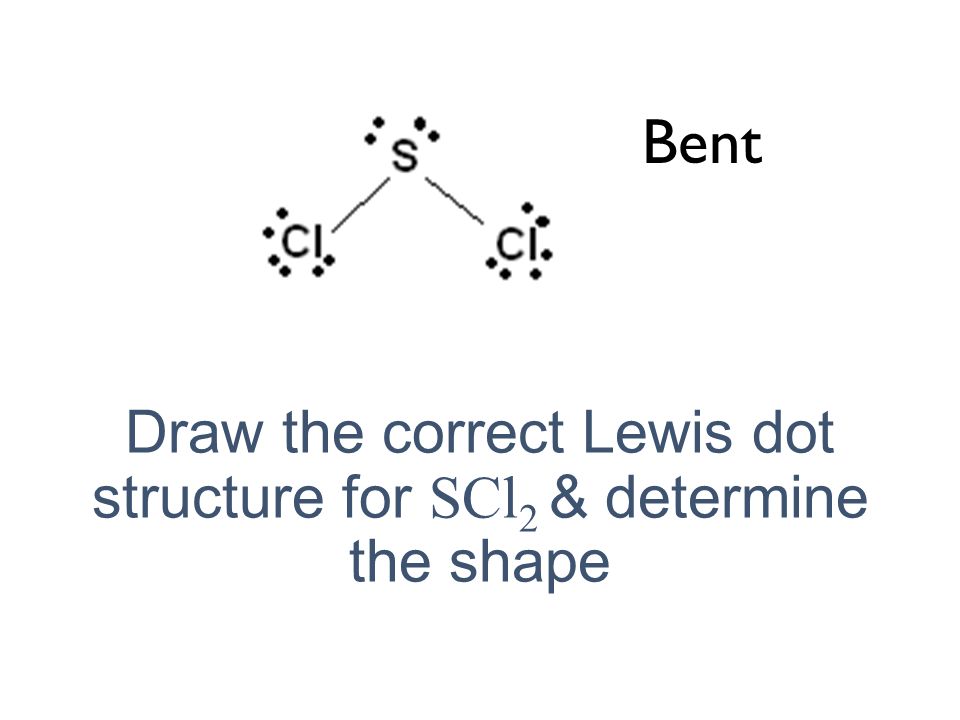
Lewis dot diagram for sodium chloride
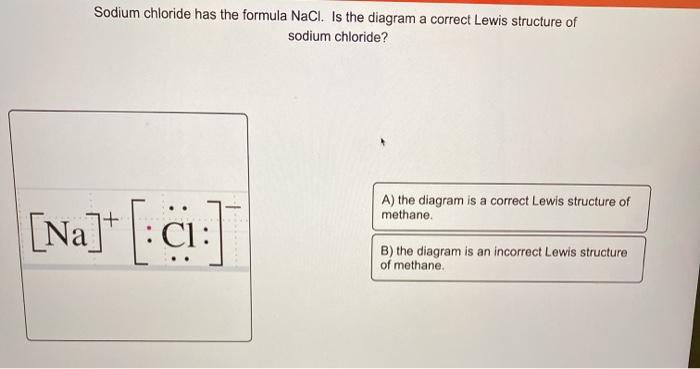
Example Question #1 : Diagrams And Geometry. Which answer option correctly depicts the Lewis dot structure of sodium chloride? Possible Answers: Correct answer: Explanation: When drawing a Lewis dot structure, we are always trying to reach an electron count where all atoms involved are stable and (usually) have full octets. 25.2.2020 · SO2 Lewis structure (sulfur dioxide electron dot structure) is that type of diagram where we show the total 18 valence electrons of SO2 as dots , or dots and dashes(-).In Lewis structure,it is common that a bonding pair of two electrons can be shown by dash(-) or dots( ) but a lone pair of two electrons is shown by dots[ ]. Free PDF download of Class 11 Chemistry revision notes & short key-notes for Chapter 4 - Chemical Bonding and Molecular Structure to score high marks in exams, prepared by expert Chemistry teachers from latest edition of CBSE(NCERT) books.
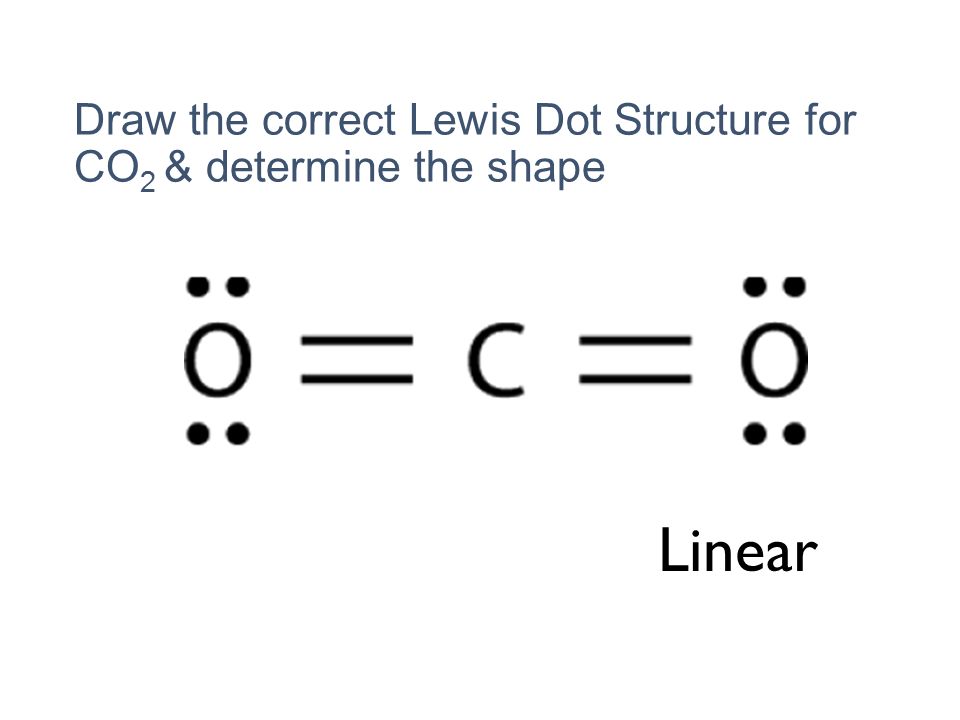
Lewis dot diagram for sodium chloride. 54 A student drew the Lewis electron-dot diagram below to represent sodium chloride. Explain why this diagram is not an accurate representation for the bonding in NaCl. [1] Answer--> Ion charges are not shown. That is a molecular structure and NaCl is ionic. Next-->Questions 55-57. Here are a number of highest rated Sodium Chloride Dot And Cross pictures on internet. We identified it from honorable source. Its submitted by processing in the best field. We endure this kind of Sodium Chloride Dot And Cross graphic could possibly be the most trending topic as soon as we portion it in google benefit or facebook. For example, consider sodium chloride. The Lewis Structure for the Salt NaCl, shows two ions which have their (Now) outer shells of electrons filled with a complete octet. In the case of the sodium cation, the filled shell is the outermost of the 'core' electron shells. ... Lewis dot diagrams give us a static picture of what the molecule or ion ... Lewis Structure Examples. The Lewis electron dot structures of a few molecules are illustrated in this subsection. 1. Lewis Structure of CO2. The central atom of this molecule is carbon. Oxygen contains 6 valence electrons which form 2 lone pairs. Since it is bonded to only one carbon atom, it must form a double bond.
(1) Electrolysis of sodium chloride brine in either diaphragm or mercury-cathode cells; chlorine is released at the anode. (2) Fused-salt electrolysis of sodium or magnesium chloride. (3) Electrolysis of hydrochloric acid. (4) Oxidation of hydrogen chloride with nitrogen oxide as catalyst and absorption of steam with sulfuric acid ("KeloChlor ... Answer: Chromium is element 24 and has electron structure [Ar]3d5 4s1, with 6 valence electrons. Chlorine is element 17 with electron configuration [Ne]3s2 3p5, with 7 valence electrons. In the +4 oxidation state, Chromium has lost 4 electrons, meaning that Chromium(IV) chloride will have the c... 1. What is the product of sodium metal reacting with chlorine gas? 2. Is energy released or absorbed in the reaction? 3. Is the reaction product (sodium chloride) more or less stable than the reactants (sodium metal and chlorine gas)? Explain. Model: Drawing Lewis Dot Structures for Atoms and Ions A. Lewis dot structure for an atom of chlorine is Learn about Chemistry, its branches, and the key concepts covered under the subject at the K-12 level. Click here to access free chemistry study material.
The Lewis dot structure for water shows the electron from hydrogen and an electron from oxygen being shared in a covalent bond. ... Ionic Bonding of Sodium Chloride. When sodium loses its only valence electron to become an ion, the Lewis structure shows it with no dots (electrons). Lewis dot diagrams are a way of drawing molecular structures while also showing valence electrons and bonds. Lewis dot diagrams serve as one of the most important topics in this unit and the course as a whole, with the ability to draw out any molecule opening the door to thousands of other possibilities. Nacl Lewis Dot Diagram. Here are a number of highest rated Nacl Lewis Dot Diagram pictures upon internet. We identified it from obedient source. Its submitted by presidency in the best field. We bow to this nice of Nacl Lewis Dot Diagram graphic could possibly be the most trending topic when we part it in google benefit or facebook. Book of Organic Chemistry . Vollhardt Organic Chemistry Structure Function 6th txtbk.PDF
Sodium carbonate, Na2CO3·10H2O, (also known as washing soda, soda ash and soda crystals) is the inorganic compound with the formula Na2CO3 and its various hydrates. What is the Lewis dot structure of Na2Co3? hey Lewis dot structure is only for covalent bonds but Na2Co3 is ionic bond so it don't have any Lewis dot structure.
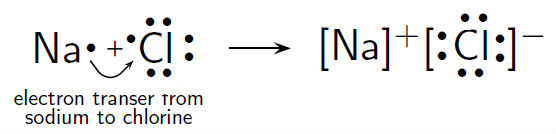
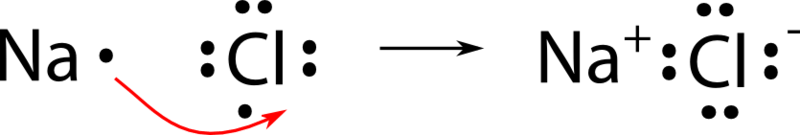
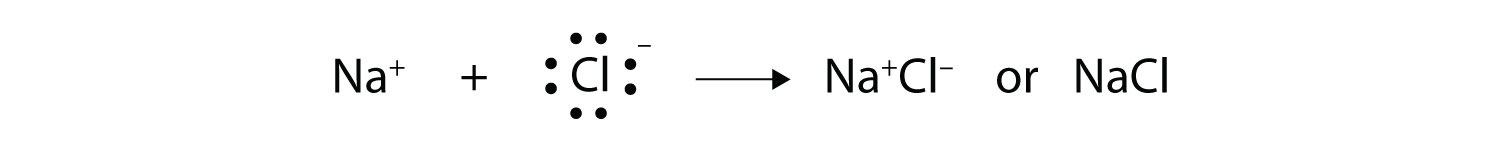
Answer: Sodium chloride is an ionic compound formed by the transfer of electrons from sodium to chlorine. Sodium atom has oxidation number of +1. Electronic configuration of sodium: Sodium atom will loose one electron to gain noble gas configuration and form sodium cation with +1 charge. What is the Lewis dot structure of beryllium?
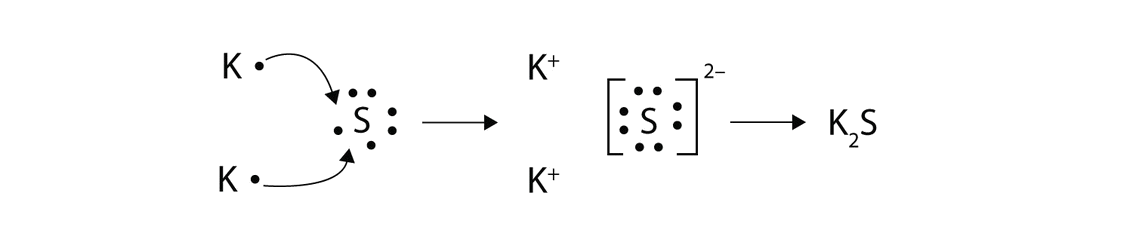
Lewis Dot Diagrams. Ionic compounds. Lithium fluoride, LiF . Aluminum chloride, AlCl. 3 . Cesium nitride, Cs. 3 N . Barium arsenide, Ba. 3 As 2. Magnesium phosphide ...
Check me out: http://www.chemistnate.com
A step-by-step explanation of how to draw the NaCl Lewis Dot Structure (Sodium chloride).For NaCl we have an ionic compound and we need to take that into acc...
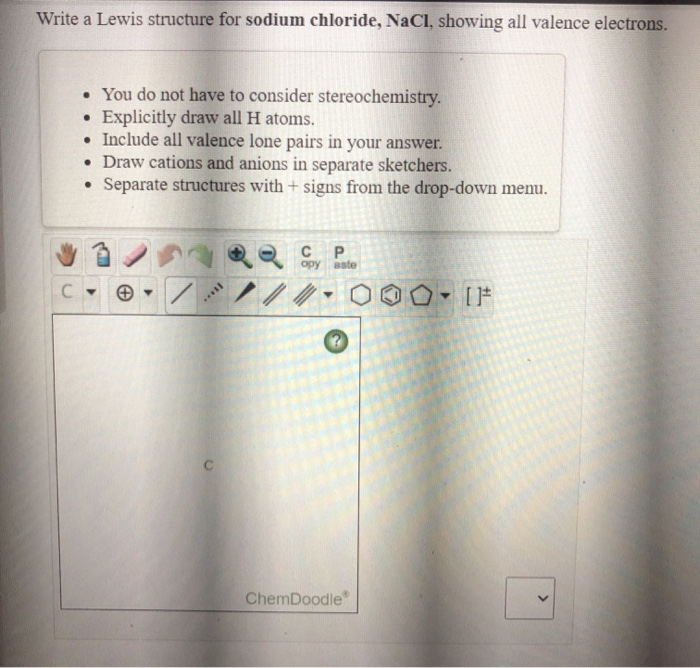
Write a Lewis structure for sodium chloride, NaCl, showing all valence electrons. . • You do not have to consider stereochemistry. Explicitly draw all H atoms. • Include all valence lone pairs in your answer. • Draw cations and anions in separate sketchers. • Separate structures with + signs from the drop-down menu. с P opy este C С ...
Using Lewis dot structures and the octet rule, we can predict and represent the electronic structure of covalently bonded molecules. For example, when two chlorine atoms, each with 7 valence electrons, come together to form a diatomic chlorine molecule, the Lewis structure shows that there will be a sharing of two electrons between the two ...
Chemical BONDING IONIC Lewis Dot Diagrams Sodium Chloride This is the finished Lewis Dot Structure [Na]+1 [ Cl ]-1 How did we get here? Practice Dot diagrams & formulas Lithium fluoride Magnesium oxide Calcium chloride Potassium hydride Drawing molecules using Lewis Dot Structures Remember: atoms are sharing e- to complete their outer shell!
Aniline is a clear to slightly yellow liquid with a characteristic odor. It does not readily evaporate at room temperature. Aniline is slightly soluble in water and mixes readily with most organic solvents. Aniline is used to make a wide variety of products such as polyurethane foam, agricultural chemicals, synthetic dyes, antioxidants, stabilizers for the rubber industry, …
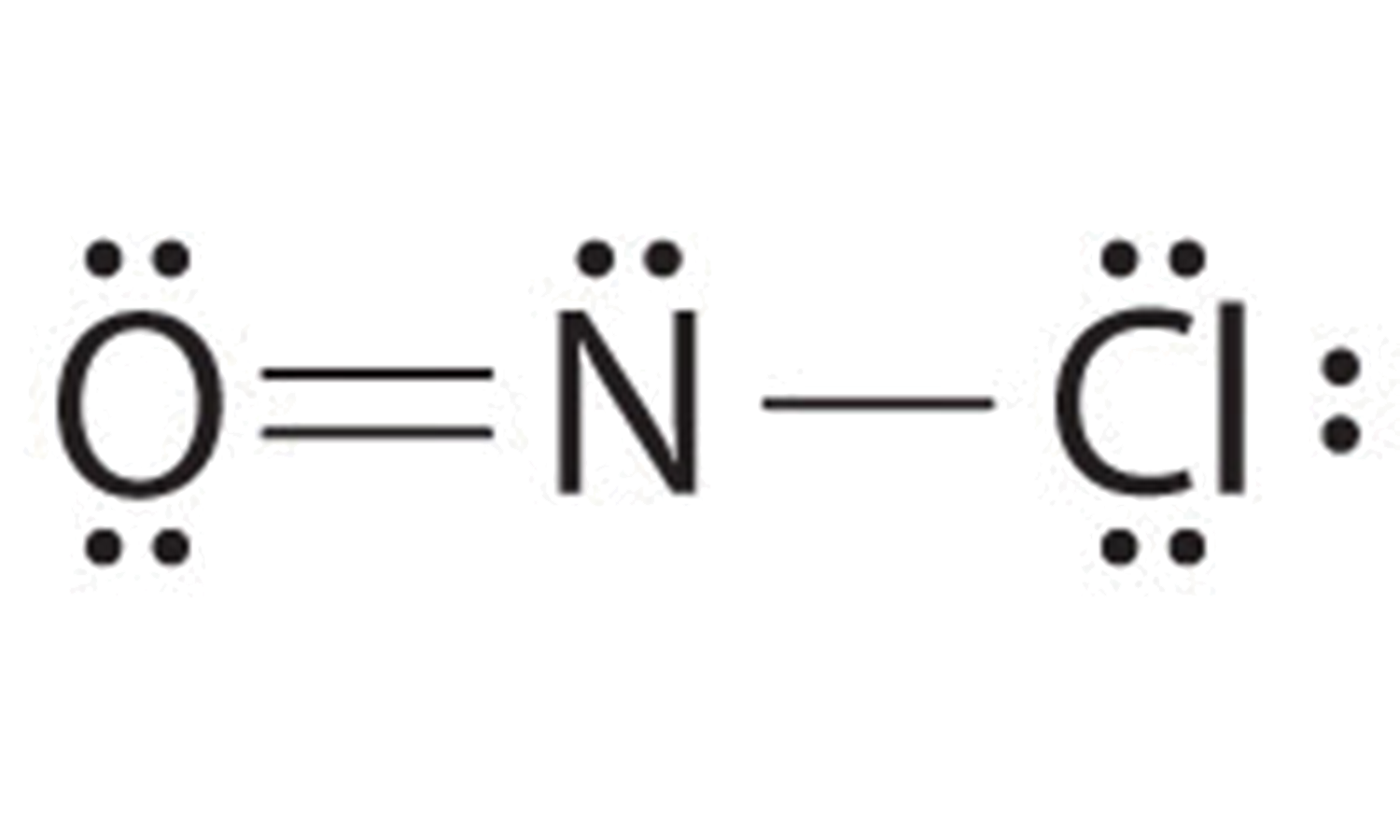
What is the correct Lewis dot structure for Sodium chloride? Lewis structure of HCN looks like... Lewis Dot Structure. diagram of a molecule using dots to represent valence electrons. octet rule. atoms react by gaining or losing electrons so as to acquire the stable electron structure of a noble gas, usually eight valence electrons.
What is the Lewis dot structure for sodium? As you can see Chlorine is now surrounded by 8 electrons in the n=3 shell and Sodium has lost its one valence electron in the n=3 shell. Of course, Sodium, is still surrounded by the 8 electrons of the n=2 shell, but we do not show electrons in the inner closed shells….Lewis Dot Structures.
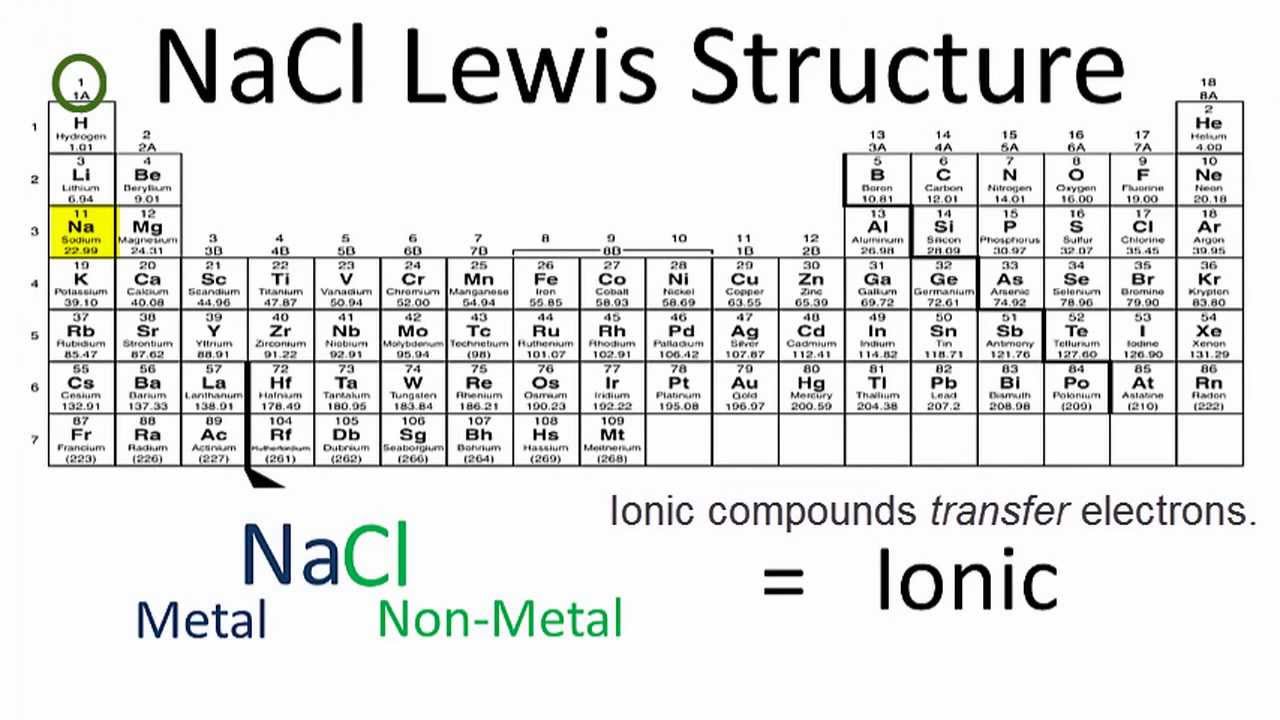
Example: sodium chloride (NaCl) Ionic bonding is common between pairs of atoms, where one of the pair is a metal of low electronegativity (such as sodium) and the second a nonmetal of high electronegativity (such as chlorine).. A chlorine atom has seven electrons in its third and outer electron shell, the first and second shells being filled with two and eight electrons respectively.
Everything you want about barium chloride lewis dot diagram will be provided by Bartendery. All information about barium chloride lewis dot diagram will always be updated with the latest, accurate.
Lewis electron-dot diagrams for CO2 and SO2 are given above. ... A particle-level diagram of a metallic element is shown above. Typically, ... aqueous solution of sodium sulfate, Na2SO4 , is a better conductor of electricity than a 0.10 M aqueous solution of sodium chloride, NaCl.
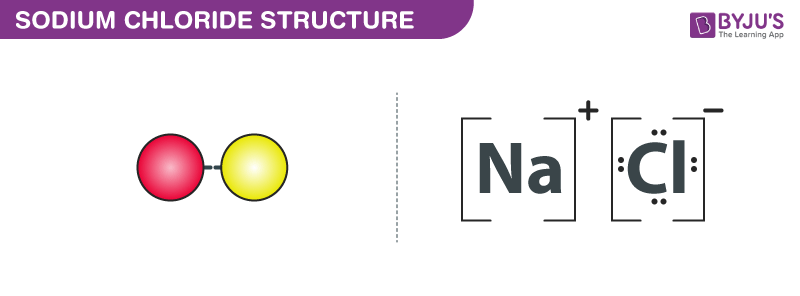
The lewis dot structure of NaCl contains one positive charge on sodium metal and one negative charge on chlorine nonmetal. We have to represent them by putting brackets around them. The lewis diagram of an ionic compound is formed with a different approach than the normal procedure we used for drawing the compounds like NH3, BF3, BrF5, etc.
The Lewis dot structure of NaCl consists of a chloride ion surrounded by eight electron dots (four pairs) and a sodium ion bonded to that chlorine ion. Typically, ionic Lewis dot structures include the ionic charge, so the Na ion is labeled +1 and Cl is labeled -1.
A. The Lewis dot structure for francium up with chlorine's unpaired dot. Chemists often depict a bond with a line, so sodium chloride can be written as Na -Cl. Draw the correct Lewis dot structure for CH2O & determine the shape Trigonal Planar. A sodium ion (2,8)+. Diagram of bonding in sodium chloride.
Sodium Chloride Dot Diagram. Here are a number of highest rated Sodium Chloride Dot Diagram pictures on internet. We identified it from reliable source. Its submitted by paperwork in the best field. We assume this nice of Sodium Chloride Dot Diagram graphic could possibly be the most trending subject later we allowance it in google benefit or ...
Na2O Lewis Structure, Uses and Properties. Sodium Oxide having a chemical formula of Na2O, is a metal oxide. It is also known as an alkali metal oxide as it comprises two sodium and one Oxygen atoms. The compound is the base anhydride for NaOH, as when Na2O reacts with water, it produces NaOH. The compound is widely used in ceramics and glasses.
Free PDF download of Class 11 Chemistry revision notes & short key-notes for Chapter 4 - Chemical Bonding and Molecular Structure to score high marks in exams, prepared by expert Chemistry teachers from latest edition of CBSE(NCERT) books.
25.2.2020 · SO2 Lewis structure (sulfur dioxide electron dot structure) is that type of diagram where we show the total 18 valence electrons of SO2 as dots , or dots and dashes(-).In Lewis structure,it is common that a bonding pair of two electrons can be shown by dash(-) or dots( ) but a lone pair of two electrons is shown by dots[ ].
Example Question #1 : Diagrams And Geometry. Which answer option correctly depicts the Lewis dot structure of sodium chloride? Possible Answers: Correct answer: Explanation: When drawing a Lewis dot structure, we are always trying to reach an electron count where all atoms involved are stable and (usually) have full octets.




![Sodium chloride]](https://www.degruyter.com/document/doi/00.0000/IUPAC.iupac.compound.5234/asset/images/5234.png)
















Comments
Post a Comment